Which Element Has the Following Configuration Xe 6s24f75d1
Thusthe electronic configuration of Eu is Xe4f 76s 2. The electrons are filled according to Afbaus rule in order of increasing energies and the electronic configuration in terms of noble gs configuration is.
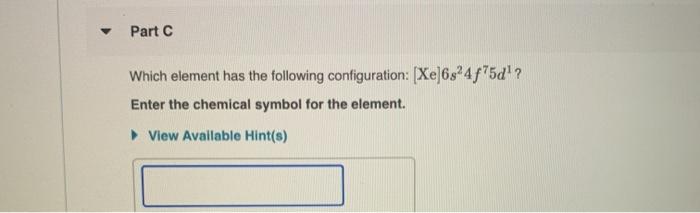
Solved Part C Which Element Has The Following Configuration Chegg Com
The element belongs to d-block of the periodic table and it will be a transition metal.
. Where the orbitals are in order by energy level. 54 7 1 2 64 electrons. Remember that the d orbitals start at.
Would the answer be d. Solution for Which element has the following configuration. Who are the experts.
The 6s and 6p electrons are in the outermost shell and are therefore the valence electrons. A ns2 b ns2 np2 c ns2 np5 d ns2. What is the quantum of light called.
Click hereto get an answer to your question The electronic configuration of four elements are. Part A What is the ground-state electron configuration of a neutral atom of manganese Express your answer in condensed form in order of increasing orbital energy. A Identify what is unusual about the electronic configurations of these elements.
You have been given the number of electrons which for the neutral element is necessarily the same as the number of. Xe has 54 electron. I Xe6s1 ii Xe4f145d16s2 iii Ar4s24p3 iv Ar3d74s2 Which one of the following statements about these elements is not correct.
Of the following which element has the highest first ionization energy. Enter the chemical symbol for the element. The element lead has the electron configuration Xe 4f14 5d10 6s2 6p2.
So far we have Kr. This formula says that lead has all the electrons of xenon as well as the electrons listed after Xe. The nearest noble gas is Xenon with 54 electrons.
This would be krypton. 100 18 ratings An alternative way to write the electron conf. The given element has electronic configuration.
Together 5425 61. Experts are tested by Chegg as specialists in their subject area. Xe 4f7 5d1 6s2.
As in it would be a noble gas with a p6 and then have a more negative e. Xe4f145d76s2 3 Ar4s24p5 4. Another way to think about it.
Simple way to get to this answer is to count the number of electron or protons in the given configuration. B Explain why these elements may have these configurations rather than the expected ones. This is the base that we use to form the configuration.
August 21 2021 thanh. Since it is in row 5 we will be filling the 5s and 5p orbitals. There are two rows usually shown below the main table labeled lanthanides and actinides.
View the full answer. Which element has the following electron configuration Xe 4f14 5d10 6s1. Consider the following neutral electron configurations in which n has a constant value.
Which element has the following configuration. The electron configuration of four elements are given below. The simplest way to identify the element is to count the electrons.
The element with 64 electrons has an atomic number 64 and that element is gadolinium Gd. Enter the chemical symbol for the element. Which element has the following electron configuration Ne 3s2 3p2.
Enter the chemical symbol for the element. Which configuration would belong to the element with the most negative electron affinity E-ea. I know its Ce but i need the electrons number too.
The metal is Iridium. Which element has the ground state electon configuration Xe6s24f75d1. The electronic configuration ends as 2 electrons in s orbital 14 electrons in f orbital and 7 electrons in d orbital.
Which element has the following configuration. Xe6s1 Write the condensed electron configurations for the following atoms using the. Total number of electrons 54214102 82.
Adding 4 more electrons to that gives 14. Which element has the following configuration Xe6s24f5. Now lets look at the row for Xenon.
Periodic Table of Elements with Electron Configuration Trends. There are a number of elements that do not follow the simple order of orbital filling. Consider the following neutral electron configurations in which n has a constant value.
Thus the electronic configuration of GdZ64 is Xe4f. Which element has the following configuration. The electronic configuration is there to distract you.
Some examples are Cr Ar4s13d5 CuAr4s13d10 and Gd Xe6s24f75d1. Ar3d74s2 Among the following statements about these elements which one statement is not correct. This problem has been solved.
An element that has the valence electron configuration 3s23p3 belongs to which period and group. A Silver b Gold c Copper d Paladium. Therefore addition of next electron doesnt occur in a more stable exactly half-filled 4f 7 shell but occur in a little higher energy 5d-orbital.
Which element has the following configuration. The element with atomic number 61 is Promethium Pm which is a Lanthanide. For facts physical properties chemical properties structure and atomic properties of the specific element click.
We review their content and use your feedback to keep the quality high. This is the best answer based on feedback and ratings. 6s orbital has 2 electron.
It glows lovely Slytherin house green color P. SiliconNeon has 10 electrons. Posted 2 years ago.
An alternative way to write the electron configuration is. The element is defined by Z the atomic number which is the number of protons positively charged massive nuclear particles. In the below periodic table you can see the trend of Electron Configuration.
So the last electron comes in d orbital. The given element is a p block element as the last electron enters the p orbital. Which configuration would belong to the element with the most negative electron affinity E-ea.
And 4f orbital has 5 electron. Z5421410282 and therefore the element is LEAD. By convention they are written last in noble gas configuration.

For Example Cr Ar 4s13d Not Ar 4s23d4 Ppt Download

Answered 1s22s22p63s1 Name The Group This Bartleby
Which Element Has The Following Configuration Xe 6s2 4f7 5d1

For Example Cr Ar 4s13d Not Ar 4s23d4 Ppt Download

Gold Facts Properties Uses Transition Metal Electron Configuration Oxidation State
Transition Metals And Coordination Chemistry
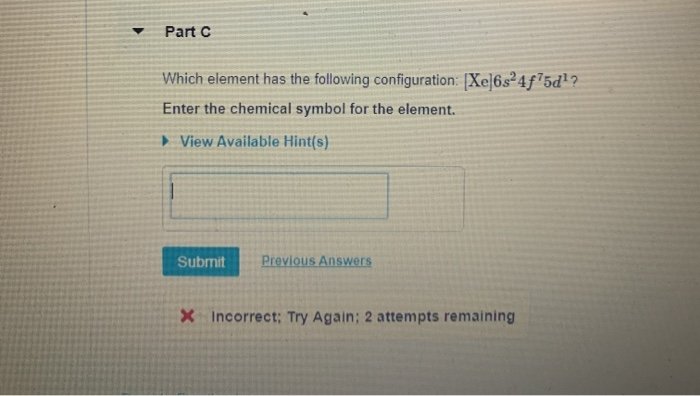
Solved Part Which Element Has The Following Configuration Chegg Com

Electron Configuration Periodic Table Quiz Quizizz

Transition Elements And Coordination Chemistry Ppt Download
Which Element Has The Following Configuration Math 54 Xe 6s 24f 5 Math Quora





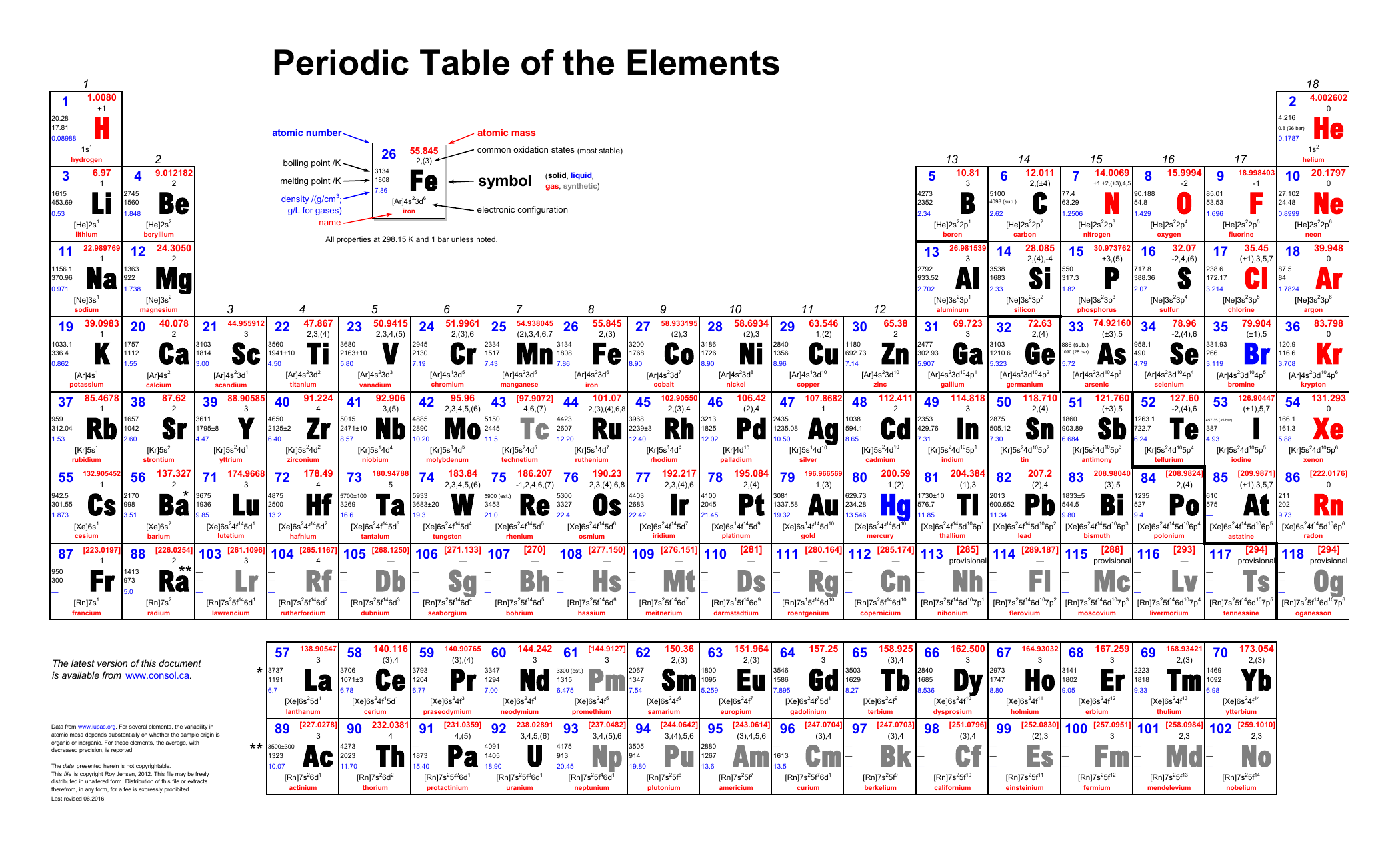

Comments
Post a Comment